Corvus Pharmaceuticals Presents Soquelitinib Preclinical Data at the Keystone Symposia on Systemic Autoimmune and Autoinflammatory Diseases
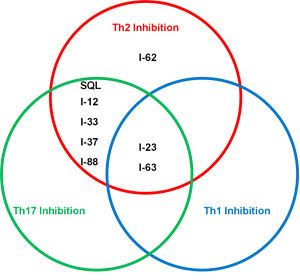
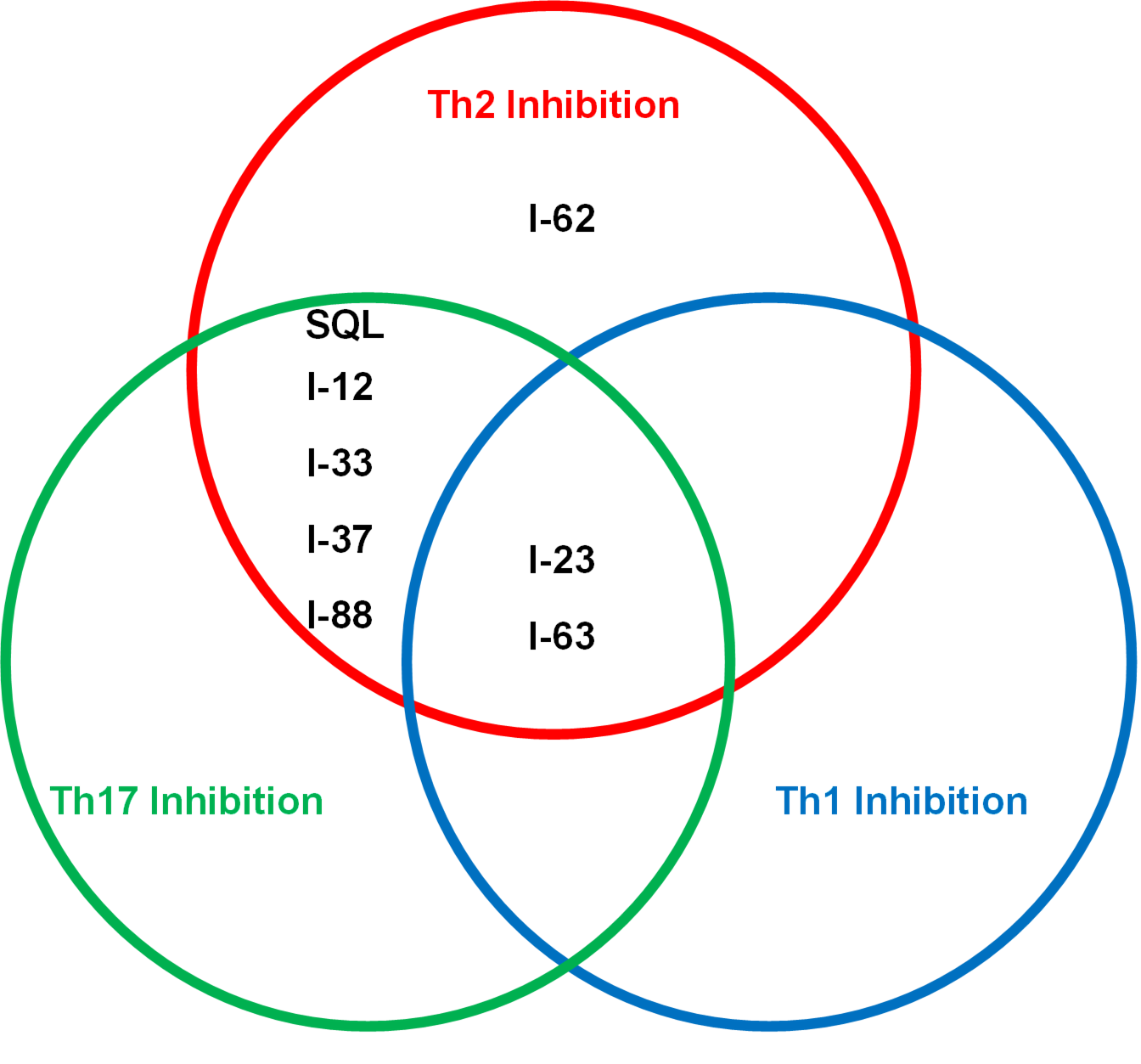
The poster presentation includes the first description of Corvus’ next-generation ITK inhibitor preclinical product candidates, which were designed to deliver precise T-cell modulation that is optimized for specific immunology indications. These preclinical product candidates exhibit specific biologic properties that are anticipated to enable more precise inhibition of Th1, Th2 and/or Th17 cell function. Atopic dermatitis (also called eczema) and asthma are thought to be mediated primarily by Th2 lymphocytes. Th17 cells are associated with psoriasis and psoriatic arthritis. A Th1 immune response is strongly implicated in rheumatoid arthritis and protection from many infections. The Venn diagram in Figure 1 illustrates the potential ability of various ITK inhibitor preclinical candidates developed by Corvus (I-number indicates individual compounds; SQL represents soquelitinib) to modify the differentiation of T cells. These results suggest that chemical structures may be refined to perform more specific biologic functions and may enable targeting of various disease types.
Figure 1: Venn diagram illustrating different biological properties of the next-gen ITK inhibitors in T cell differentiation assays.
“We have established our leadership in ITK inhibition with the development of soquelitinib, which is advancing on track towards the initiation of a registration Phase 3 trial in relapsed peripheral T cell lymphoma and the initiation of a randomized, placebo-controlled Phase 1 trial in atopic dermatitis,” said
The preclinical data demonstrate that soquelitinib was active in six different models of T cell-mediated inflammatory and immune disease, including acute and chronic asthma, pulmonary fibrosis, systemic sclerosis (scleroderma), psoriasis, and acute graft versus host disease. This activity was shown to be the result of soquelitinib’s ability to impact disease-associated cytokines by targeting the cellular sources, specifically Th2 and Th17 cells, which produce various cytokines including IL-4, IL-5, IL-13 and IL-17. Some of the data from the poster presentation were recently published online in a preprint at bioRxiv.org.
The soquelitinib preclinical data and information on the Company’s next-generation ITK inhibitor candidates was presented by
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company’s lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Corvus plans to initiate a Phase 3 registrational clinical trial for soquelitinib in patients with relapsed peripheral T cell lymphoma. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com.
About Soquelitinib
Soquelitinib (formerly known as CPI-818) is an investigational small molecule drug given orally designed to selectively inhibit ITK (interleukin-2-inducible T cell kinase), an enzyme that is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. The immunologic effects of soquelitinib lead to what is known as Th1 skewing and is made possible by the high selectivity of soquelitinib for ITK. Research on soquelitinib’s mechanism of action suggests that it has the potential to control differentiation of normal T helper cells and enhance immune responses to tumors by augmenting the generation of cytotoxic killer T cells and the production of cytokines that inhibit cancer cell survival. Soquelitinib has also been shown to prevent T cell exhaustion, a major limitation of current immunotherapy and CAR-T therapies. Optimal doses of soquelitinib have been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of their secreted cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases. Based on interim results from a Phase 1/1b clinical trial in patients with refractory T cell lymphomas, which demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, the Company plans to initiate a registrational Phase 3 clinical trial of soquelitinib in patients with relapsed PTCL.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of the Company’s product candidates including soquelitinib, ciforadenant and mupadolimab; the potential use of soquelitinib to treat a variety of solid tumors, hematological cancers, as well as autoimmune and allergic diseases; and the potential of the Company’s next-generation ITK inhibitor preclinical product candidates. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the three months ended September 30, 2023, filed with the Securities and Exchange Commission on
INVESTOR CONTACT:
Chief Financial Officer
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/4536b195-3481-4fb9-9bd3-db24486aafbd

Source: Corvus Pharmaceuticals, Inc.