Corvus Pharmaceuticals Announces Interim Data from Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
Data from lowest dose level cohorts demonstrate a favorable safety and efficacy profile
Data includes complete results from cohort 1 and initial results from cohort 2
Early exercise of common stock warrants from stockholder generates cash proceeds of approximately
Company to host conference call and webcast today at 8:00 a.m. ET / 5:00 a.m. PT
“We are pleased with the early results of our soquelitinib Phase 1 atopic dermatitis clinical trial, which show an attractive potential product profile at the lowest dose we are studying,” said
Soquelitinib Atopic Dermatitis Phase 1 Clinical Trial Design
The randomized, double-blind, placebo-controlled Phase 1 clinical trial is planned to enroll 64 patients with moderate to severe atopic dermatitis that previously failed one prior topical or systemic therapy. Patients are enrolled into one of four dosing cohorts in a 3:1 ratio (12 active and 4 placebo) to receive either soquelitinib or placebo. The cohorts are sequentially enrolled and will examine 100 mg oral twice per day, 200 mg oral once per day, 200 mg oral twice per day and 400 mg oral once per day. Patients are treated for 28 days and are then followed for an additional 30 days with no therapy.
These doses were selected based on the Company’s prior experience evaluating soquelitinib in T cell lymphoma patients. The doses in the atopic dermatitis trial bracket the 200 mg oral twice a day dosing regimen, which is the level that has been shown to provide complete ITK occupancy and that is being evaluated in the Company’s ongoing registrational Phase 3 clinical trial of soquelitinib in peripheral T cell lymphoma.
The primary endpoints include safety and tolerability, and efficacy, measured by improvement in Eczema Area and Severity Index (EASI) score, Investigator Global Assessment (IGA), reduction in itch and various cytokine biomarkers.
Soquelitinib Interim Data from the Atopic Dermatitis Phase 1 Clinical Trial
The Company is reporting complete results from Cohort 1 of the trial, which includes 16 patients (12 that received soquelitinib 100 mg oral twice per day and four that received placebo) with follow up at 28 days and at 58 days. At 58 days, two patients in the soquelitinib group were not available for follow up. The soquelitinib and placebo patients were well matched; see Table 1 below for patient characteristics.
| Table 1: Cohort 1 Patient Characteristics | ||
| Soquelitinib | Placebo | |
| (N=12) | (N=4) | |
| Age, mean (range), yrs | 46.3 (30–66) | 50.5 (32–62) |
| Gender, male n (%) | 7 (58.3) | 4 (100) |
| Race/ethnicity, n (%) | ||
| Asian | 2 (16.7) | 0 (0) |
| Black or |
6 (50) | 4 (100) |
| White | 3 (25) | 0 (0) |
| Hispanic or Latino | 1 (8.3) | 0 (0) |
| Baseline |
20.4 (15.0–46.6) | 18.5 (14.9–24.8) |
| Baseline IGA, mean (range) | 3.0 (2–4) | 3.3 (3–4) |
| Prior AD therapies, n (%) | ||
| Topical Corticosteroids | 11 (91.7) | 4 (100) |
| Systemic therapies | 3 (25) | 2 (50) |
| Concomitant topical steroids | 0 (0) | 1 (25) |
- The mean baseline
EASI and IGA scores for soquelitinib patients were 20.4 and 3.0, respectively, compared to anEASI score of 18.5 and an IGA score of 3.3 for placebo patients. - All soquelitinib patients discontinued topical corticosteroids prior to enrollment, while one placebo patient continued topical corticosteroid treatment. All patients, except the one placebo, discontinued topical corticosteroids for at least 27 days prior to enrolling in the study.
- Cohort 1 included a high proportion of
African American patients: 50% of the soquelitinib group and 100% of the placebo group. African Americans with atopic dermatitis are known to have a less favorable prognosis compared to other patient populations.
Cohort 1 Efficacy Data
The cohort 1
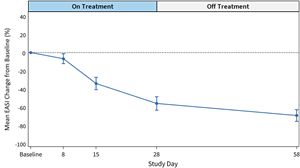
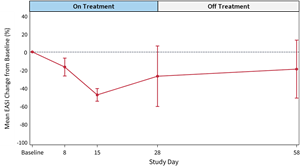
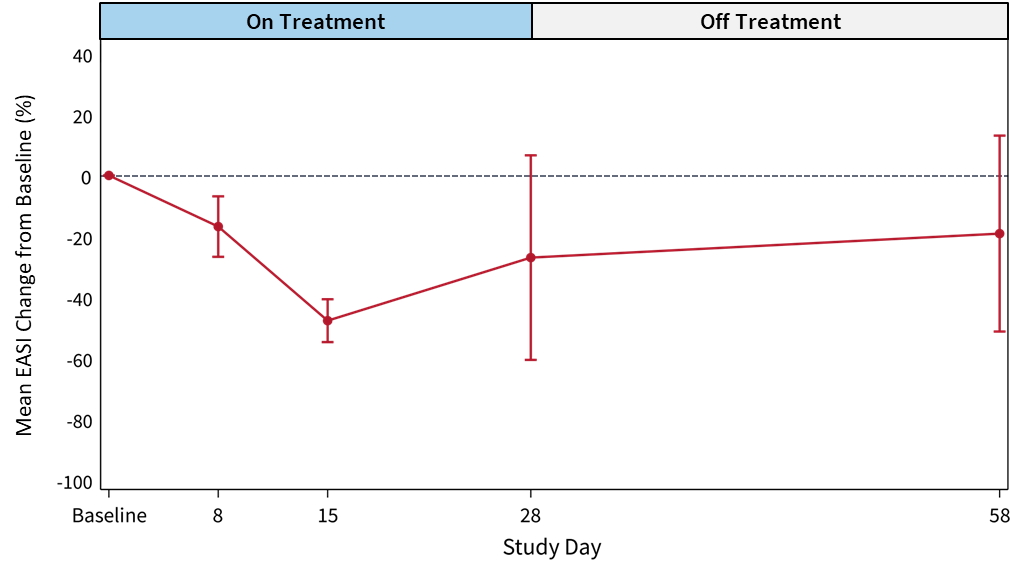
EASI scores at 28-day and 58-day follow-up demonstrate a favorable effect of soquelitinib treatment compared to placebo.- The soquelitinib mean
EASI score reduction was 55.9% at 28 days (n=12) compared to meanEASI reduction of 27.0% in placebo. At day 58, continued improvement in the soquelitinib group was seen with meanEASI reduction of 69.1% (n=10) compared to meanEASI reduction of 19.1% for the placebo group. - At day 28, in the soquelitinib group, nine of 12 patients achieved
EASI 50; three of 12 achievedEASI 75 and one of 12 achievedEASI 90. Three of 12 patients achieved IGA 0 or 1. In the placebo group, two of four patients achievedEASI 50 and no patients achievedEASI 75,EASI 90 or IGA 0 or 1. - At day 58, in the soquelitinib group, nine of 10 patients achieved
EASI 50, four of 10 achievedEASI 75 and one of 10 achievedEASI 90. Three of 10 patients achieved IGA 0 or 1. In the placebo group, one in four patients achievedEASI 50 and no patients achievedEASI 75,EASI 90 or IGA 0 or 1. - The timing of
EASI improvement in the soquelitinib group indicates that a treatment effect begins early, at eight days, and continues for the remainder of the study. (See Figures 1 and 2 below). All soquelitinib treated patients showed improvement inEASI scores. - The small number of placebo patients demonstrates a variable course over the treatment period with no substantial change over the 58-day period.
| Table 2: Cohort 1 Efficacy Results | ||||
| 4 week (Day 28) | 8 week (Day 58) | |||
| Placebo | Soquelitinib | Placebo | Soquelitinib | |
| (N=4) | (N=12) | (N=4) | (N=10) | |
| Change Mean % Reduction |
27.0 | 55.9 | 19.1 | 69.1 |
| 50 | 75 | 25 | 90 | |
| 0 | 25 | 0 | 40 | |
| 0 | 8 | 0 | 10 | |
| IGA 0 or 1 (%pts) | 0 | 25 | 0 | 30 |
Figure 1: Mean EASI Score Change from Baseline (%) for
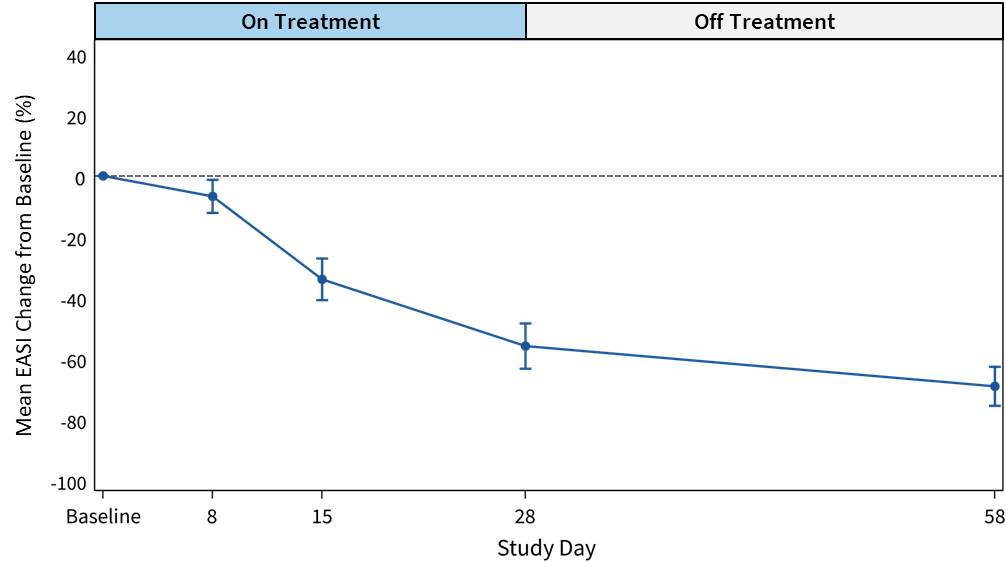
Figure 2: Mean EASI Score Change from Baseline (%) for
Cohort 1 Safety Data
No significant safety issues were observed. All the patients completed 28 days of dosing. One patient reported Grade 1 nausea that did not interfere with the subject receiving the full treatment course, and one patient developed COVID-19 on day 28 of treatment; that patient had an uneventful recovery. No clinically significant laboratory abnormalities were seen. See Table 3 below.
| Table 3: Cohort 1 Safety | ||
| Soquelitinib | Placebo | |
| (N=12) | (N=4) | |
| Subjects with adverse events | 2* | 0 |
| Serious adverse events | 0 | 0 |
| Adverse events leading to study drug discontinuation | 0 | 0 |
| Adverse events leading to death | 0 | 0 |
| Treatment-related adverse events: | ||
| Nausea (Grade 1) | 1 | 0 |
| *Reported adverse events: Nausea (N=1) and Covid-19 (N=1); both resolved without any dose modification. | ||
Serum Cytokine Changes
Relationships between reductions in certain cytokines with improvement in
Cohort 2 Initial Efficacy and Safety Data
As of
Early Exercise of Common Stock Warrants
The Company also announced that
Conference Call, Webcast and Presentation Slides
Corvus will host a conference call and webcast today, Wednesday, December 18, 2024 from 8:00 – 9:00 a.m. ET to provide an overview of the soquelitinib atopic dermatitis Phase 1 clinical data. The conference call can be accessed by dialing 1-800-717-1738 (toll-free domestic) or 1-646-307-1865 (international) or by clicking on this link for instant telephone access to the event. The live webcast, which will include presentation slides, may be accessed via the investor relations section of the Corvus website. A replay of the webcast will be available on Corvus’ website for 60 days.
About Atopic Dermatitis
Atopic dermatitis, also called eczema, is a chronic disease that can cause inflammation, redness, scaly patches, blisters and irritation of the skin. It affects up to 20% of children and up to 10% of adults, and treatments include topical therapies, oral therapies and systemic injectable biologic therapies. It is frequently associated with other allergic disorders such as food allergies and asthma. Atopic dermatitis, like asthma and allergy, involves the participation of Th2 lymphocytes which secrete cytokines that result in inflammation. Soquelitinib has been shown in preclinical studies to inhibit cytokine production from Th2 lymphocytes.
About Soquelitinib
Soquelitinib (formerly CPI-818) is an investigational small molecule drug given orally designed to selectively inhibit ITK (interleukin-2-inducible T cell kinase), an enzyme that is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. Soquelitinib has been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of their secreted cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases. Recent studies have demonstrated that ITK controls a switch between the differentiation of Th17 proinflammatory cells and T regulatory suppressor cells. Inhibition of ITK leads to a shift toward T regulatory cell differentiation which has the potential to suppress autoimmune and inflammatory reactions. Based on interim results from a Phase 1/1b clinical trial in patients with refractory T cell lymphomas, which demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, the Company has initiated a registrational Phase 3 clinical trial (NCT06561048) of soquelitinib in patients with relapsed PTCL. Soquelitinib is also now being investigated in a randomized placebo-controlled phase 1 clinical trial in patients with atopic dermatitis. A recent publication describing the chemistry, enzymology and biology of soquelitinib appeared in npj Drug Discovery in
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company’s lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of the Company’s product candidates including soquelitinib; the potential use of soquelitinib to treat atopic dermatitis and the potential of ITK inhibition as a novel mechanism of action for other immune diseases; and the Company’s conduct of, enrollment in and timing of clinical trials, including the Company’s Phase 3 clinical trial in PTCL and Phase 1 clinical trial in atopic dermatitis. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the three months ended
INVESTOR CONTACT:
Chief Financial Officer
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
Photos accompanying this announcement are available at
https://www.globenewswire.com/NewsRoom/AttachmentNg/b695c700-155b-4d6a-80eb-8bbcd1b8886e
https://www.globenewswire.com/NewsRoom/AttachmentNg/f1496538-062f-4dc0-ac1c-0a935bf23cd9

Source: Corvus Pharmaceuticals, Inc.