Corvus Pharmaceuticals Announces Data from Cohort 2 of Placebo-Controlled Phase 1 Clinical Trial of Soquelitinib for Atopic Dermatitis
“As we increase the number of patients, we remain excited by the outlook for our Phase 1 clinical trial of soquelitinib for the treatment of atopic dermatitis,” said
Soquelitinib New Interim Data from the Atopic Dermatitis Phase 1 Clinical Trial
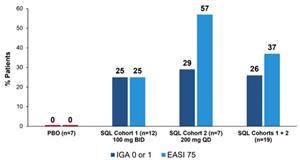
The Company is reporting results from 16 patients in Cohort 1 (12 patients in the soquelitinib group receiving 100 mg orally twice per day vs. four receiving placebo) and 10 patients in Cohort 2 (seven patients in the soquelitinib group receiving 200 mg orally once per day vs. three receiving placebo) for which 28 days of treatment have been completed. For those 19 patients in the soquelitinib group, 26% achieved IGA 0 or 1 and 37% achieved
No significant safety issues were observed and no clinically significant laboratory abnormalities were seen. All 10 patients from Cohort 2 completed 28 days of dosing at the full dose of 200 mg orally once per day; the remaining patients from Cohort 2 are at various stages of treatment. Cohort 2 of the trial is fully enrolled (N=16) and the Company plans to report full results from all four study cohorts in the second quarter 2025.
Figure 1: Percent Patients Achieving Endpoints IGA 0 or 1,
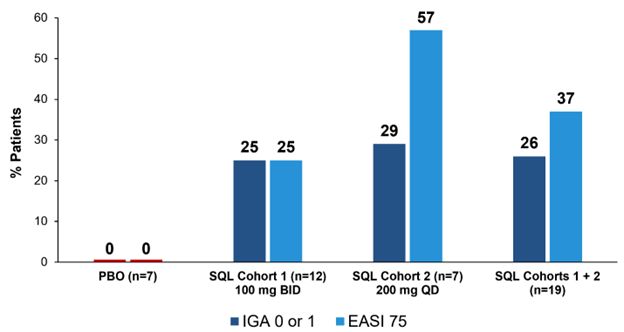
The placebo patients from Cohort 1 (n=4) and Cohort 2 (n=3) are combined.
Soquelitinib Atopic Dermatitis Phase 1 Clinical Trial Design
The randomized, double-blind, placebo-controlled Phase 1 clinical trial is planned to enroll 64 patients with moderate to severe atopic dermatitis that previously failed one prior topical or systemic therapy. Patients are enrolled into one of four dosing cohorts in a 3:1 ratio (12 active and four placebo) to receive either soquelitinib or placebo. The cohorts are sequentially enrolled and will examine 100 mg orally twice per day, 200 mg orally once per day, 200 mg orally twice per day and 400 mg orally once per day. Patients are treated for 28 days and are then followed for an additional 30 days with no therapy.
These doses were selected based on the Company’s prior experience evaluating soquelitinib in T cell lymphoma patients. The doses in the atopic dermatitis trial studied in Cohorts 1 and 2 are lower than the 200 mg orally twice a day dosing regimen, which is the level that has been shown to provide complete ITK occupancy and that is being evaluated in the Company’s ongoing registrational Phase 3 clinical trial of soquelitinib in peripheral T cell lymphoma.
The primary endpoints include safety and tolerability. Efficacy, measured by improvement in
Upcoming Presentation and Webcast
About Atopic Dermatitis
Atopic dermatitis, also called eczema, is a chronic disease that can cause inflammation, redness, scaly patches, blisters and irritation of the skin. It affects up to 20% of children and up to 10% of adults, and treatments include topical therapies, oral therapies and systemic injectable biologic therapies. It is frequently associated with other allergic disorders such as food allergies and asthma. Atopic dermatitis, like asthma and allergy, involves the participation of Th2 lymphocytes which secrete cytokines that result in inflammation. Soquelitinib has been shown in preclinical studies to inhibit cytokine production from Th2 lymphocytes.
About Soquelitinib
Soquelitinib (formerly CPI-818) is an investigational small molecule drug given orally designed to selectively inhibit ITK (interleukin-2-inducible T cell kinase), an enzyme that is expressed predominantly in T cells and plays a role in T cell and natural killer (NK) cell immune function. Soquelitinib has been shown to affect T cell differentiation and induce the generation of Th1 helper cells while blocking the development of both Th2 and Th17 cells and production of their secreted cytokines. Th1 T cells are required for immunity to tumors, viral infections and other infectious diseases. Th2 and Th17 helper T cells are involved in the pathogenesis of many autoimmune and allergic diseases. The Company believes the inhibition of specific molecular targets in T cells may be of therapeutic benefit for patients with cancers, including solid tumors, and in patients with autoimmune and allergic diseases. Recent studies have demonstrated that ITK controls a switch between the differentiation of Th17 proinflammatory cells and T regulatory suppressor cells. Inhibition of ITK leads to a shift toward T regulatory cell differentiation which has the potential to suppress autoimmune and inflammatory reactions. Based on interim results from a Phase 1/1b clinical trial in patients with refractory T cell lymphomas, which demonstrated tumor responses in very advanced, refractory, difficult to treat T cell malignancies, the Company has initiated a registrational Phase 3 clinical trial (NCT06561048) of soquelitinib in patients with relapsed PTCL. Soquelitinib is also now being investigated in a randomized placebo-controlled phase 1 clinical trial in patients with atopic dermatitis. A recent publication describing the chemistry, enzymology and biology of soquelitinib appeared in npj Drug Discovery in
About Corvus Pharmaceuticals
Corvus Pharmaceuticals is a clinical-stage biopharmaceutical company pioneering the development of ITK inhibition as a new approach to immunotherapy for a broad range of cancer and immune diseases. The Company’s lead product candidate is soquelitinib, an investigational, oral, small molecule drug that selectively inhibits ITK. Its other clinical-stage candidates are being developed for a variety of cancer indications. For more information, visit www.corvuspharma.com.
Forward-Looking Statements
This press release contains forward-looking statements, including statements related to the potential safety and efficacy of the Company’s product candidates including soquelitinib; the outlook for the Phase 1 clinical trial of soquelitinib; physician and patient receptivity to soquelitinib; the potential use of soquelitinib to treat atopic dermatitis and other immune diseases; the Company’s conduct of, enrollment in and timing of clinical trials and results, including the Company’s Phase 3 clinical trial in PTCL and Phase 1 clinical trial in atopic dermatitis; and the potential of ITK inhibition as a new approach to immunotherapy. All statements other than statements of historical fact contained in this press release are forward-looking statements. These statements often include words such as “believe,” “expect,” “anticipate,” “intend,” “plan,” “estimate,” “seek,” “will,” “may” or similar expressions. Forward-looking statements are subject to a number of risks and uncertainties, many of which involve factors or circumstances that are beyond the Company’s control. The Company’s actual results could differ materially from those stated or implied in forward-looking statements due to a number of factors, including but not limited to, risks detailed in the Company’s Quarterly Report on Form 10-Q for the three months ended
INVESTOR CONTACT:
Chief Financial Officer
+1-650-900-4522
llea@corvuspharma.com
MEDIA CONTACT:
Real Chemistry
+1-949-903-4750
sseapy@realchemistry.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/789edc2f-f3d8-4ffc-80c2-41765cceff02

Source: Corvus Pharmaceuticals, Inc.